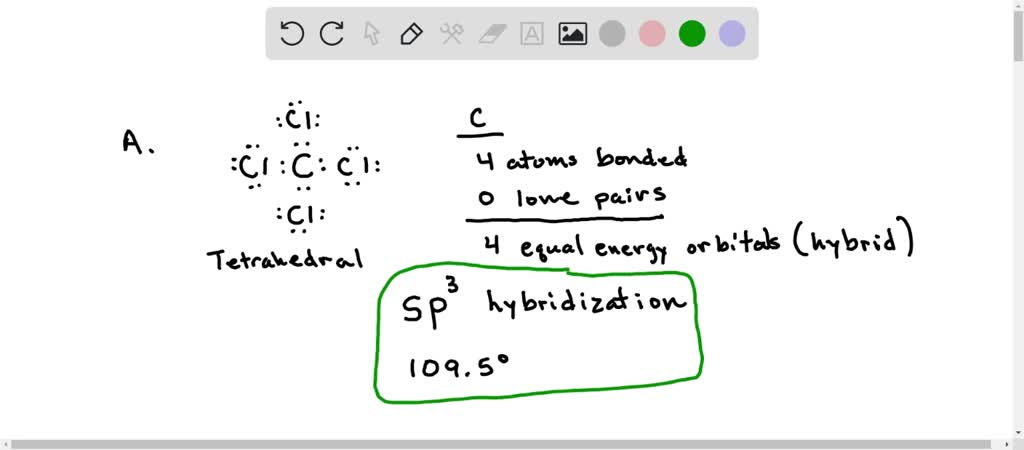
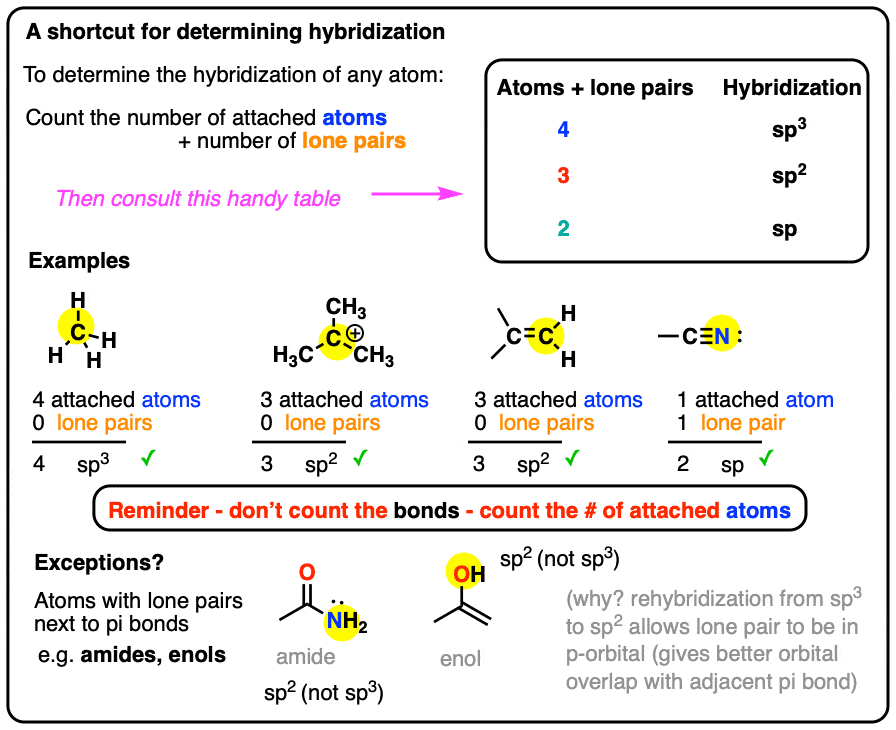
SOLVED: A. What is the hybridization of the central atom in CCl4? Hybridization = What are the approximate bond angles in this substance? Bond angles = ° B. What is the hybridization

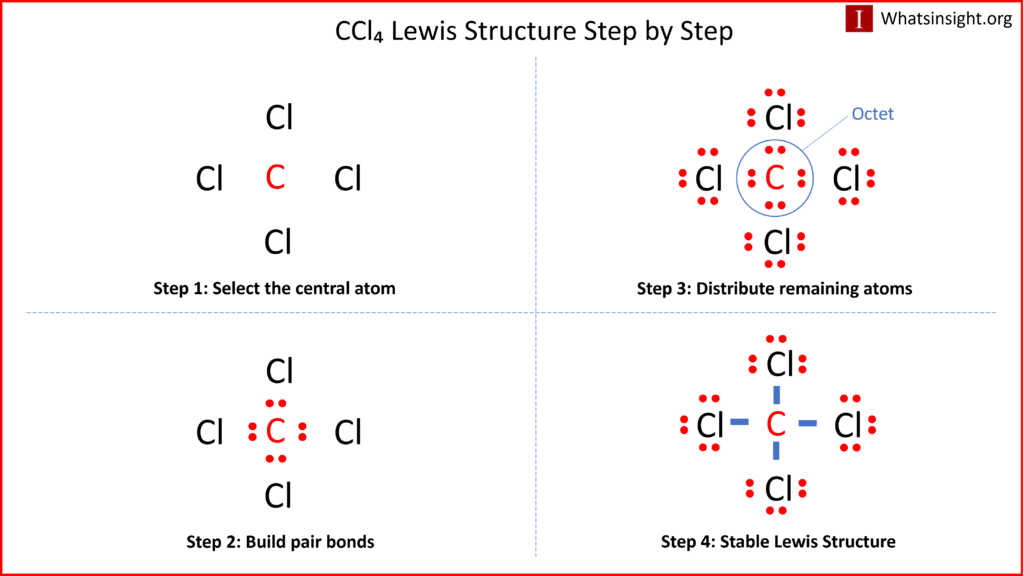
SOLVED: The hybridization of the carbon atom in the carbon tetrachloride molecule (CCl4) is of the type sp3.
We all know that CCl4 and SiCl4 both compound remains in Tetrahedral structure , with a hybridization of sp³ in the central atom. Now , Si is a much larger atom than

Draw the Lewis dot structure for CCl4. Determine the electron geometry and molecular shape of this molecule. Is this molecule polar or nonpolar? | Homework.Study.com
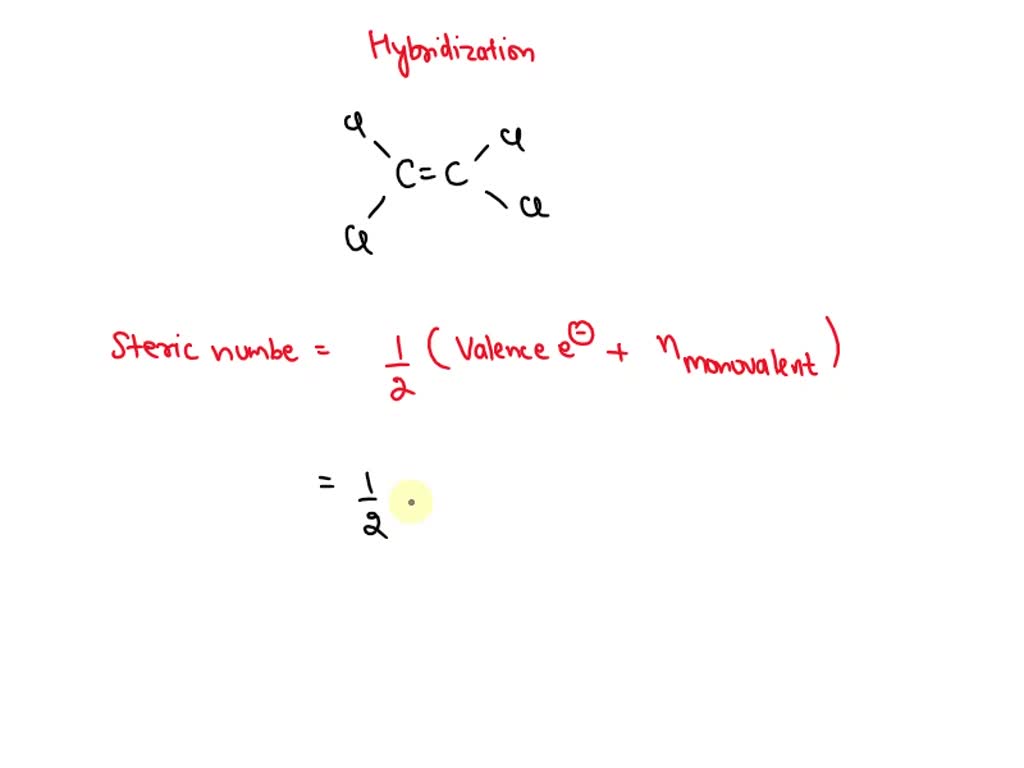
SOLVED: Tetrachloroethylene, a common dry-cleaning solvent, has the formula CCl4. Its structure is C=C-Cl. Use the VSEPR and VB theories to describe the bonding in this molecule. C hybridization: sp3. The double
Chemokine-mediated inflammation in the degenerating retina is coordinated by Müller cells, activated microglia, and retinal pigment epithelium | Journal of Neuroinflammation | Full Text

![ANSWERED] What is the hybridization of the central atom in CCl₄? What - Kunduz ANSWERED] What is the hybridization of the central atom in CCl₄? What - Kunduz](https://media.kunduz.com/media/sug-question/raw/76551348-1658842026.6477146.jpeg)


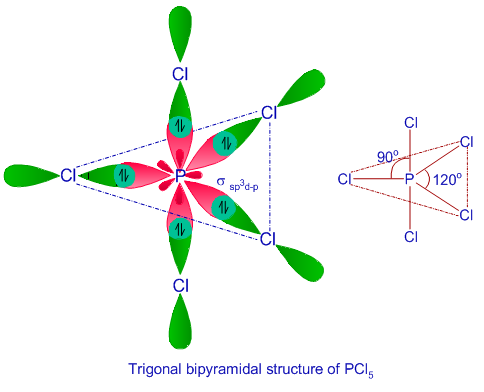






![Explain hybridization of central atoms in \\[CC{l_4}\\]. Explain hybridization of central atoms in \\[CC{l_4}\\].](https://www.vedantu.com/question-sets/1e78619c-a1a6-4268-a0bc-9c654e3103565131807361122774649.png)

