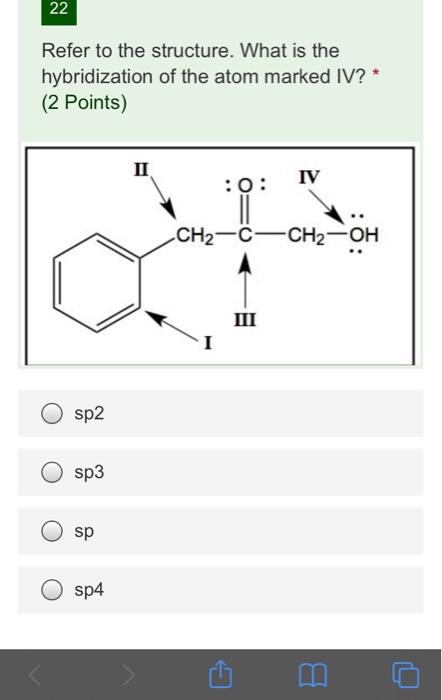
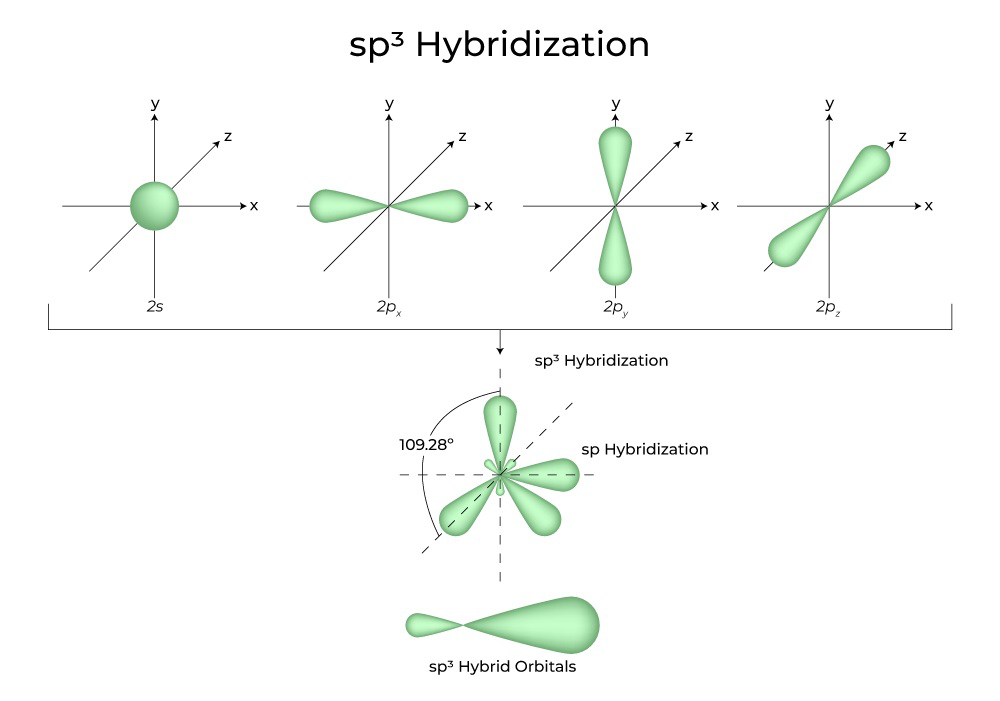
Draw an orbital picture of allene, H2C=C=CH2. What hybridization must the central carbon atom have to form two double bonds? What shape does allene have? | Homework.Study.com
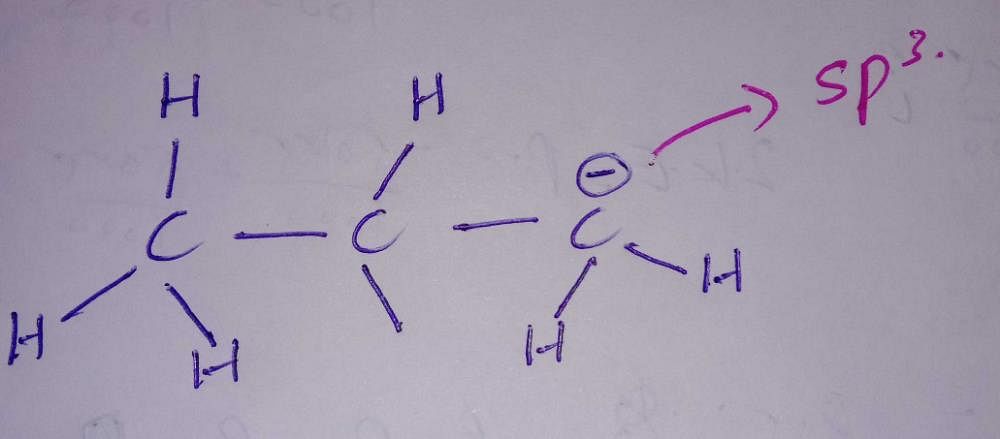
Hybridisation of the third carbon in CH3-CH2-CH2 (-) ( There's negative charge in third carbon) ? - EduRev NEET Question

Prepare a sketch of the molecule CH3CCl = CH2 showing orbital overlaps. Identify the type of hybridization of atomic orbitals on each carbon atom. | Homework.Study.com

What is the hybridization of the atoms from left to right? H2C=C=CH-CH2-CH= CH2 | Homework.Study.com
Write the type of hybridisation of each of the carbon atom in the following structures: (i) ch2=c=ch2 (ii) ch3 ch=ch ch3
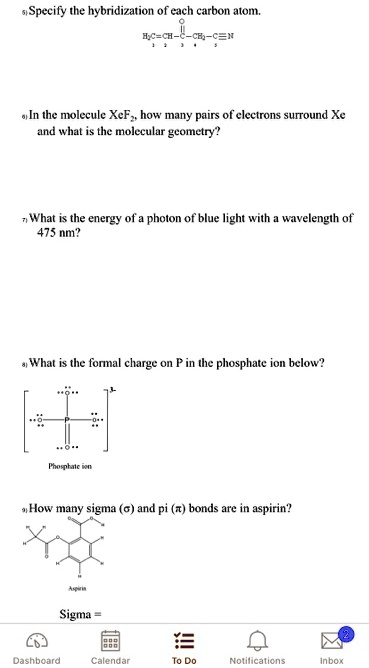



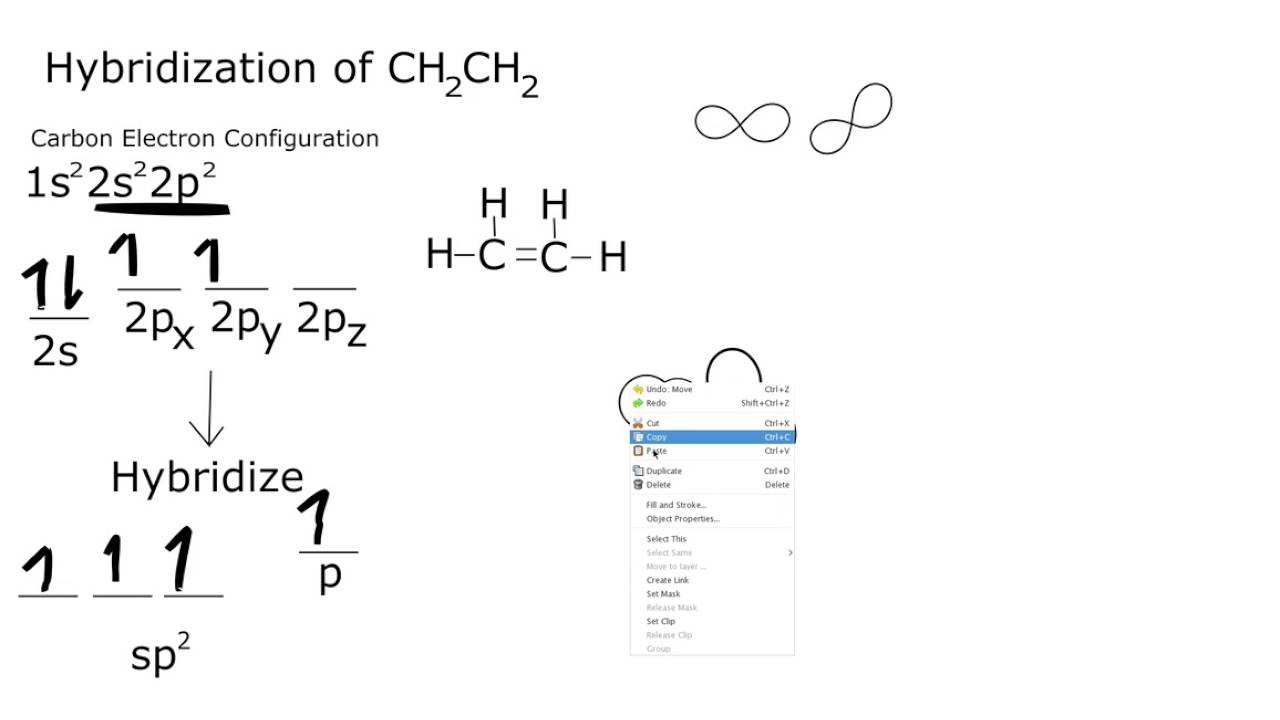
.jpg?revision=1&size=bestfit&width=601&height=192)