
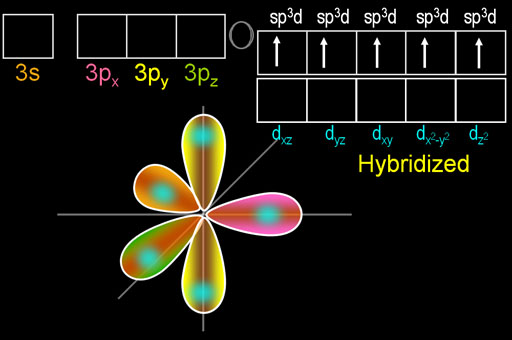
organic chemistry - How to explain shape of molecules in penta and hexa coordination if hybridization involving d-orbitals (in main block) is considered incorrect? - Chemistry Stack Exchange

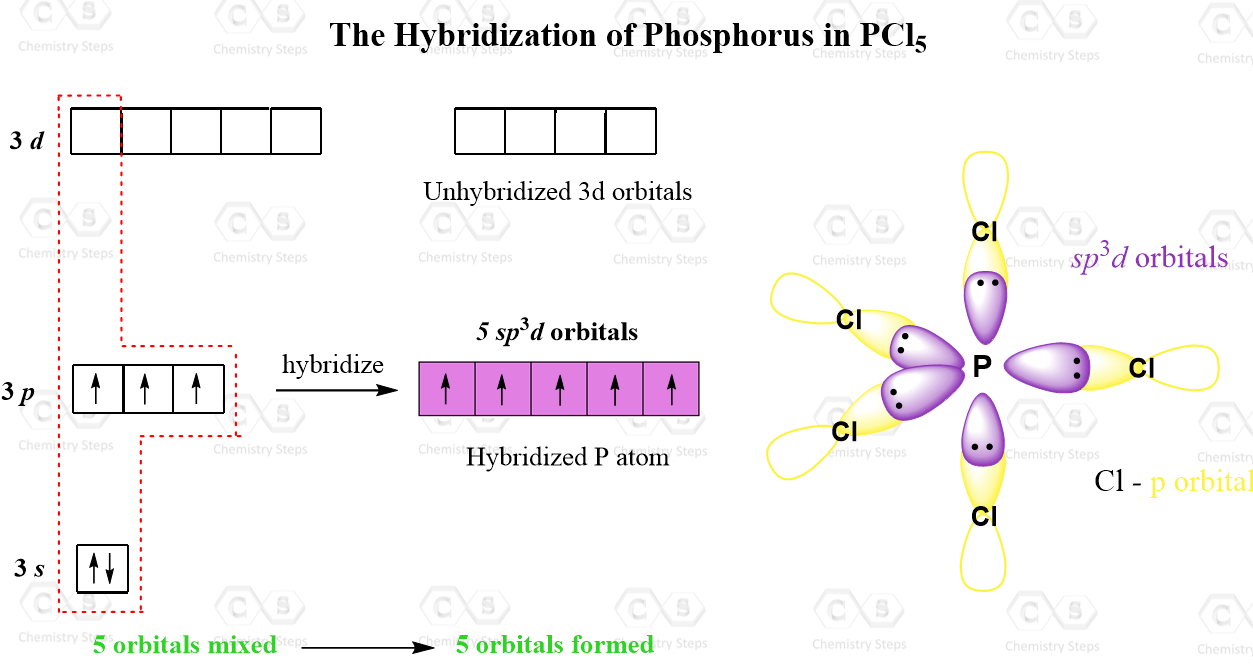
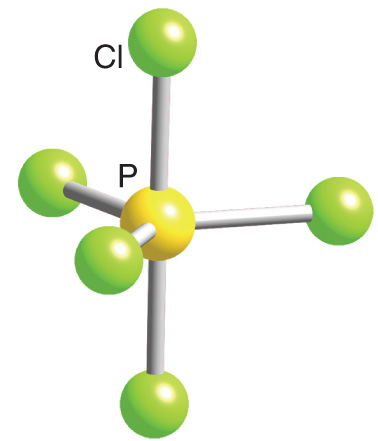
Describe the hybridization in the case of PCl5 ? Why are the axial bonds longer compared to the equatorial bonds?
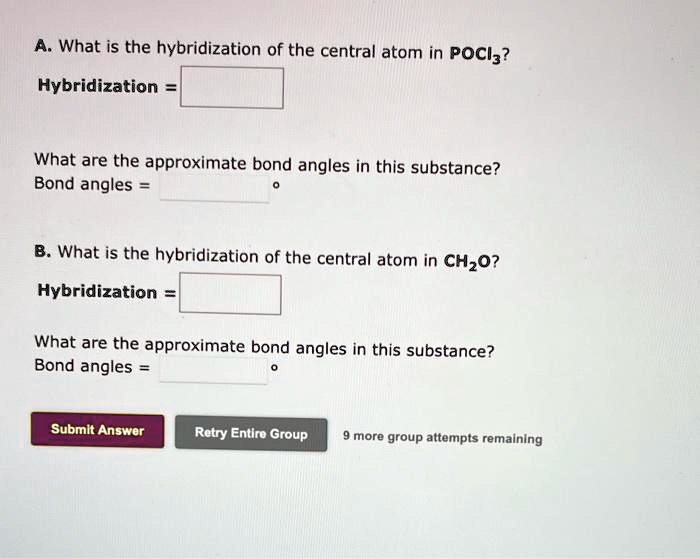
SOLVED: A. What is the hybridization of the central atom in PCl5? Hybridization= What are the approximate bond angles in this substance? Bond angles= B. What is the hybridization of the central
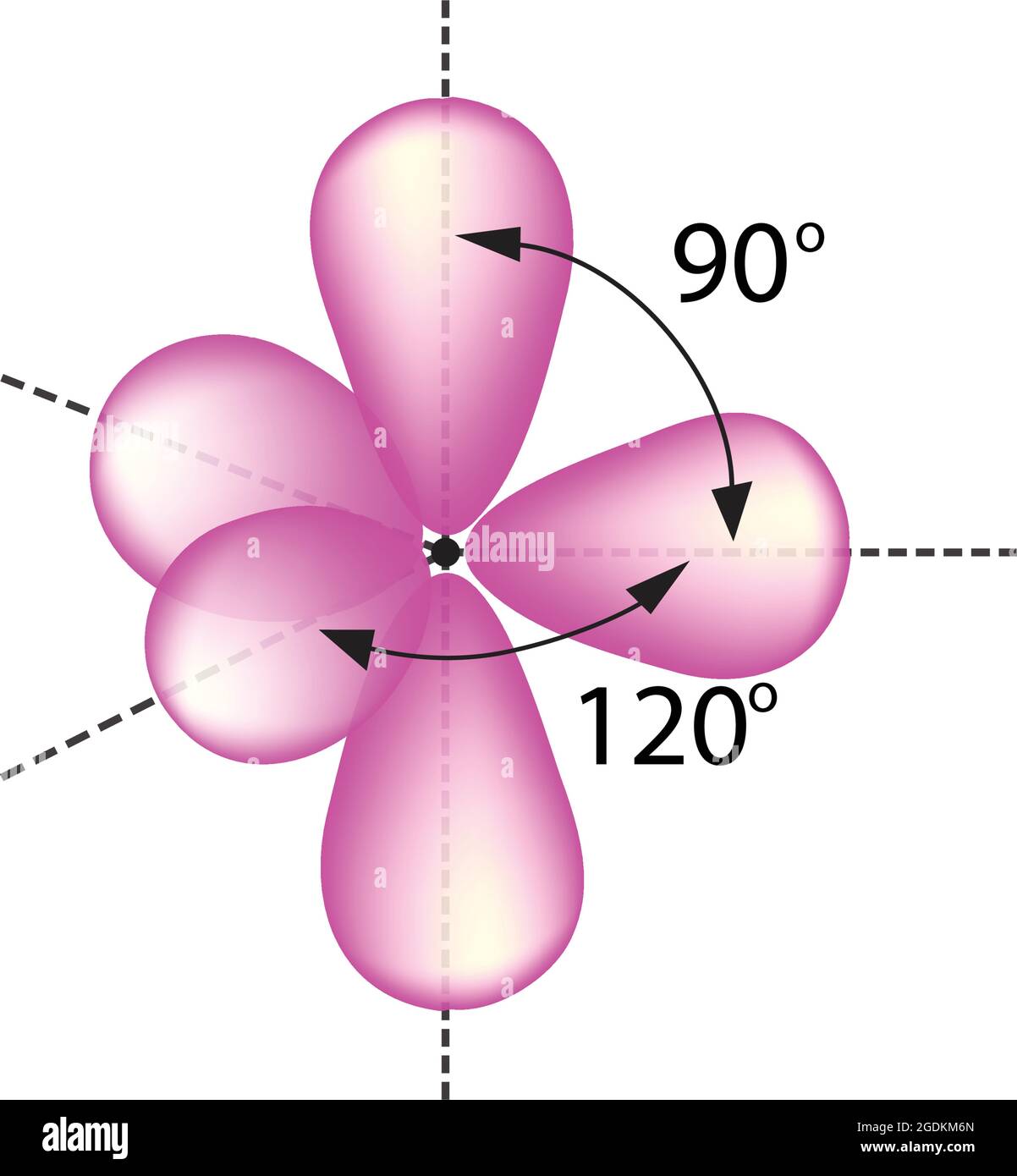
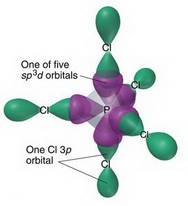
Trigonal bipyramidal arrangement of hybridization, 5 sp3d hybrid orbitals. Three orbitals are arranged around the equator of the molecules, PCl5, SbF5 Stock Vector Image & Art - Alamy

Define Hybridisation State the hybridization the shape of PCl5 and BeF2 - Chemistry - Chemical Bonding and Molecular Structure - 16996831 | Meritnation.com
![P in \\[PC{l_5}\\] has \\[s{p^3}\\] hybridization. Which one of the following statement is wrong about \\[PC{l_5}\\] structure? A.Two \\[P - Cl\\] bonds are stronger and three \\[P - Cl\\] bonds are weaker.B.Two \\[ P in \\[PC{l_5}\\] has \\[s{p^3}\\] hybridization. Which one of the following statement is wrong about \\[PC{l_5}\\] structure? A.Two \\[P - Cl\\] bonds are stronger and three \\[P - Cl\\] bonds are weaker.B.Two \\[](https://www.vedantu.com/question-sets/f467e8e6-90f5-4bd7-a209-34d55cc126f55014518861279383844.png)



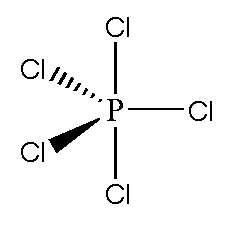

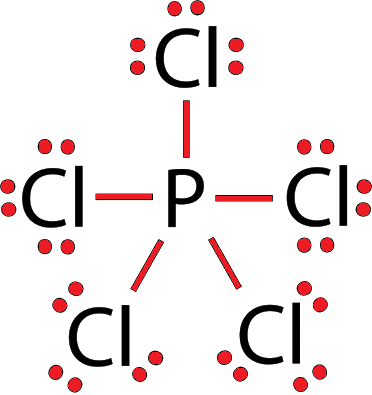


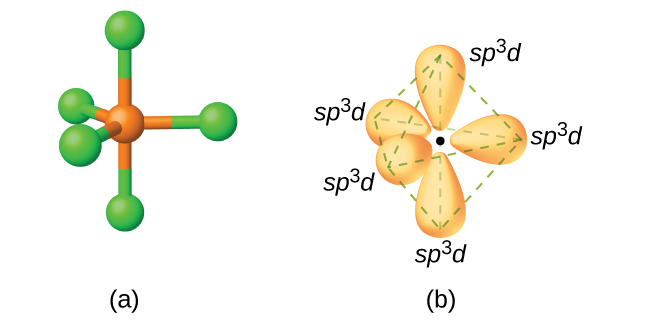



![Solved] The PCl5 molecule has trigonal bipyramidal structure. T Solved] The PCl5 molecule has trigonal bipyramidal structure. T](https://storage.googleapis.com/tb-img/production/22/02/dsp3.png)