
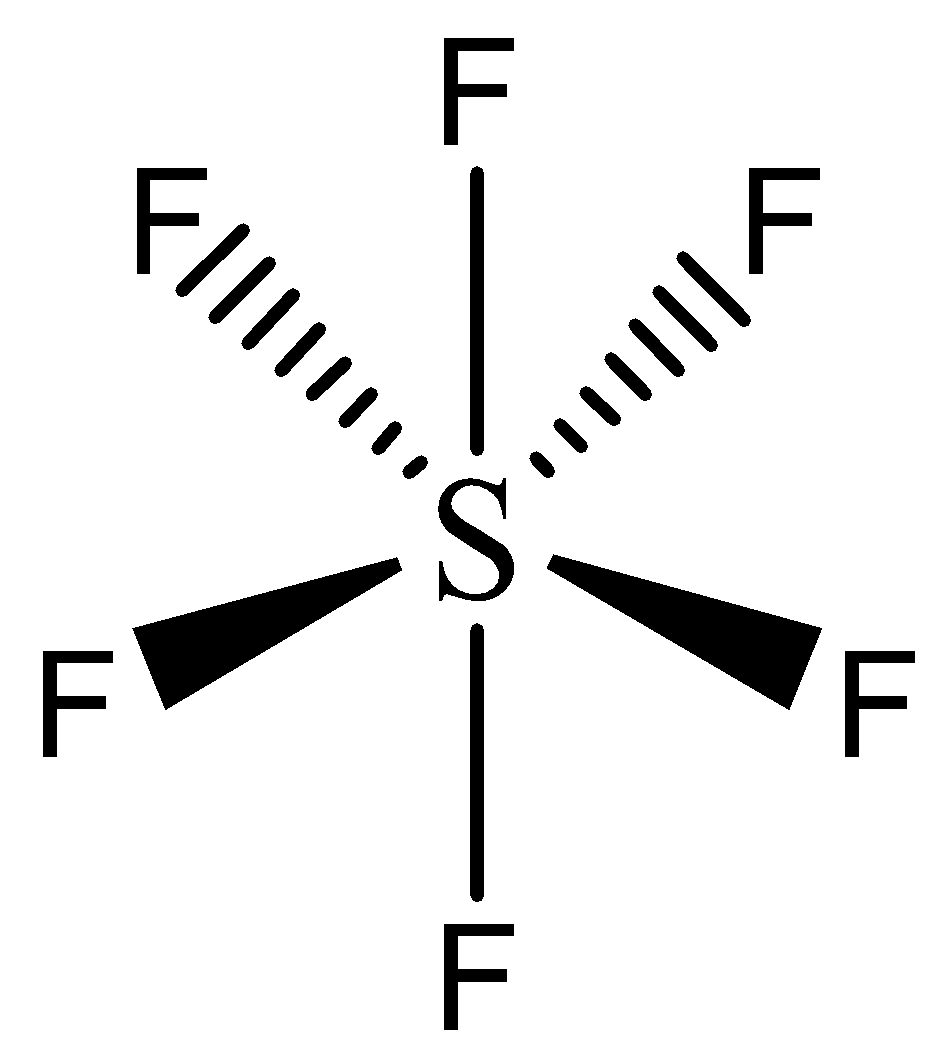
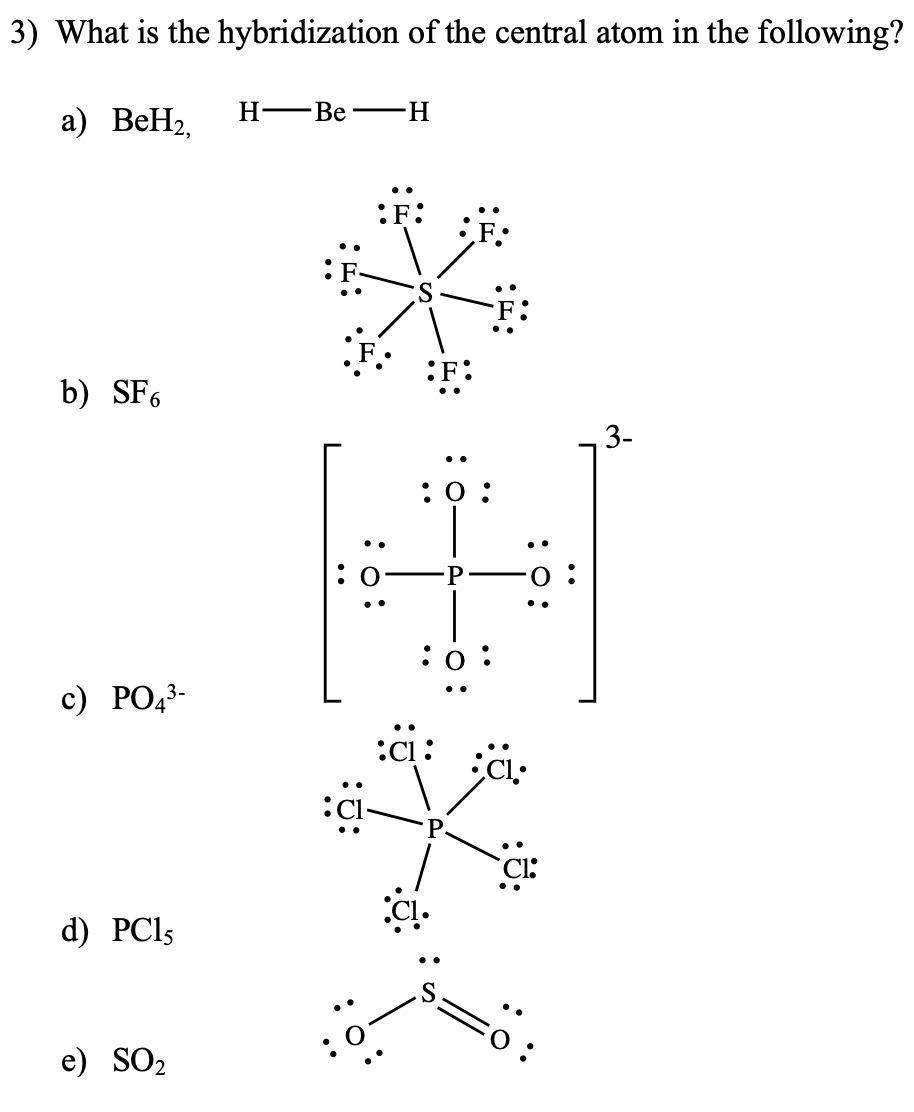
Describe hybridisation in the case of PCl5 and SF6. The axial bonds are longer as compared to equatorial bonds in PCl5 whereas in SF6 axial bonds and equatorial bonds have the same

What is the hybridisation of SF6 whats the method to find it - Chemistry - Chemical Bonding and Molecular Structure - 5272961 | Meritnation.com

Write the electronic configuration and hybridization of SF6 molecule - Chemistry - - 15171799 | Meritnation.com

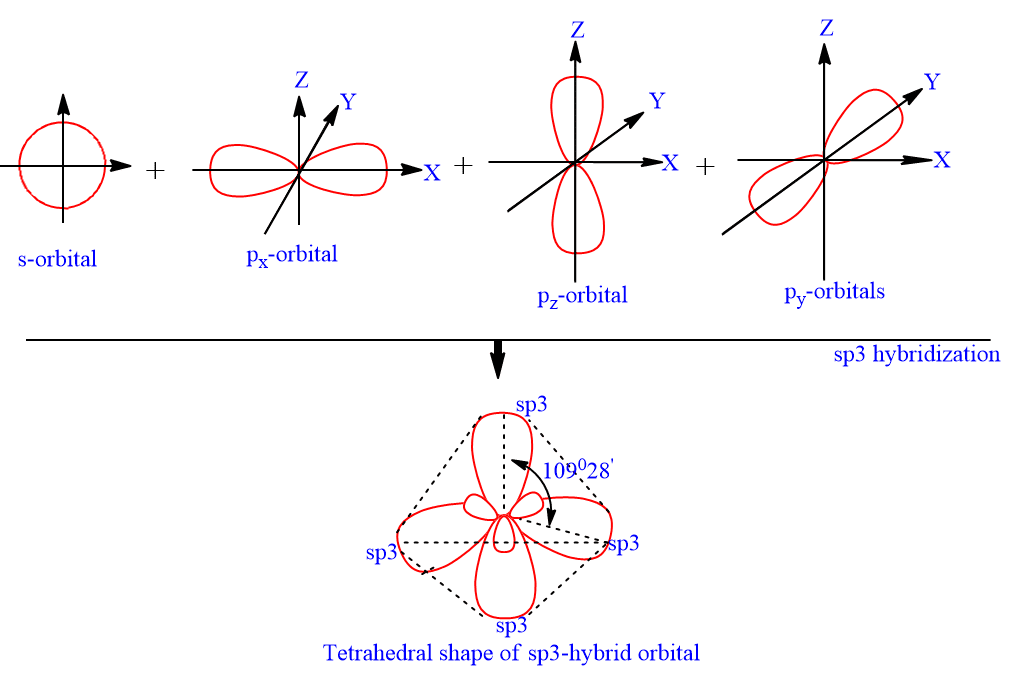
The sp3d2 Hybridization and Octahedral Geometry | Molecular geometry, Chemistry, Electron configuration

Chemistry - Molecular Structure (35 of 45) s-p3-d2 Hybridization - Sulfur Hexafloride - SF6 - YouTube



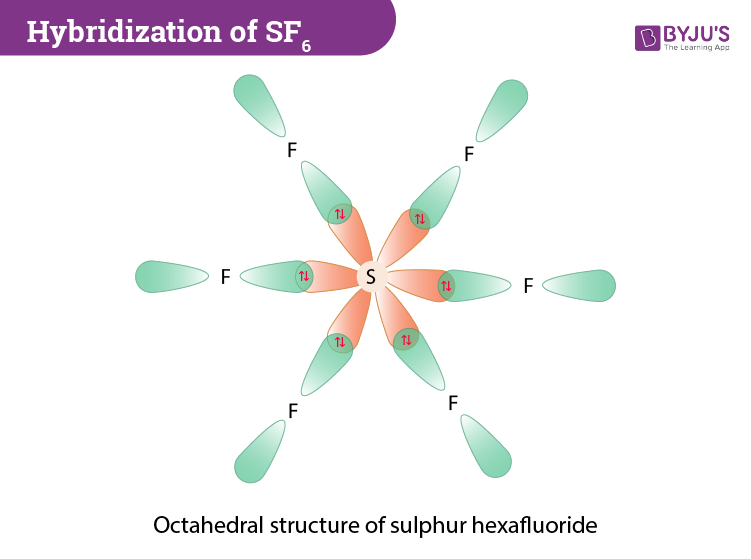
![Solved] In SF6 the hybridisation of sulphur is: Solved] In SF6 the hybridisation of sulphur is:](https://storage.googleapis.com/tb-img/production/21/01/F1_Utkarsha_15.1.21_Pallavi_D28.png)


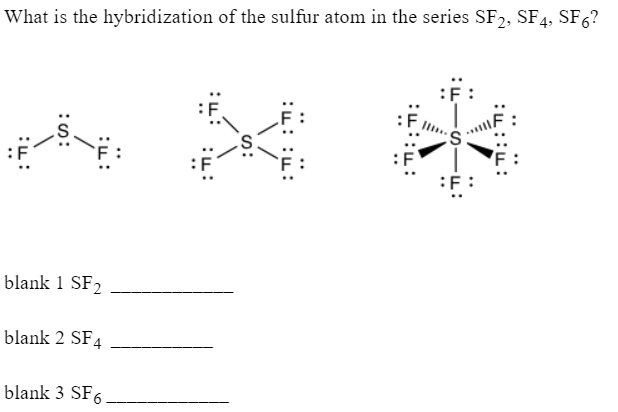
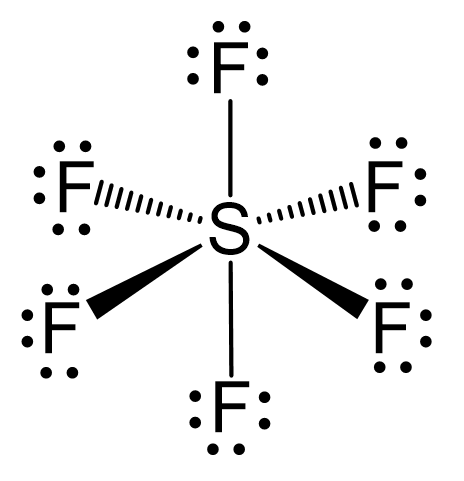



![Solved] In SF6 the hybridisation of sulphur is: Solved] In SF6 the hybridisation of sulphur is:](https://storage.googleapis.com/tb-img/production/21/01/F1_Utkarsha_15.1.21_Pallavi_D27.png)

