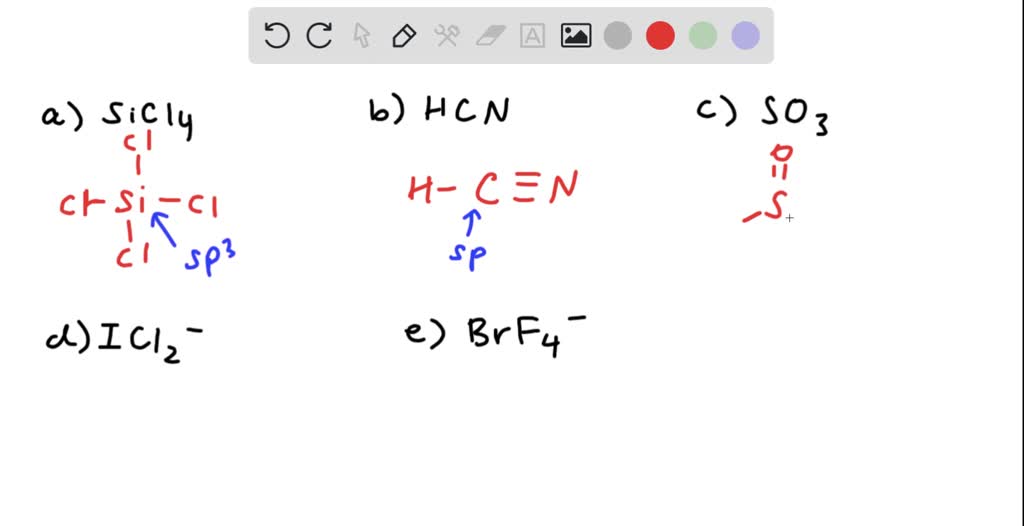
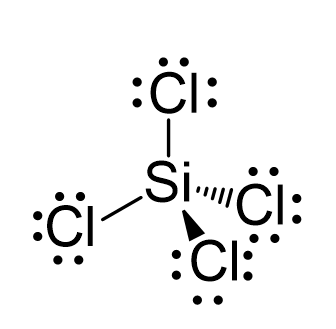
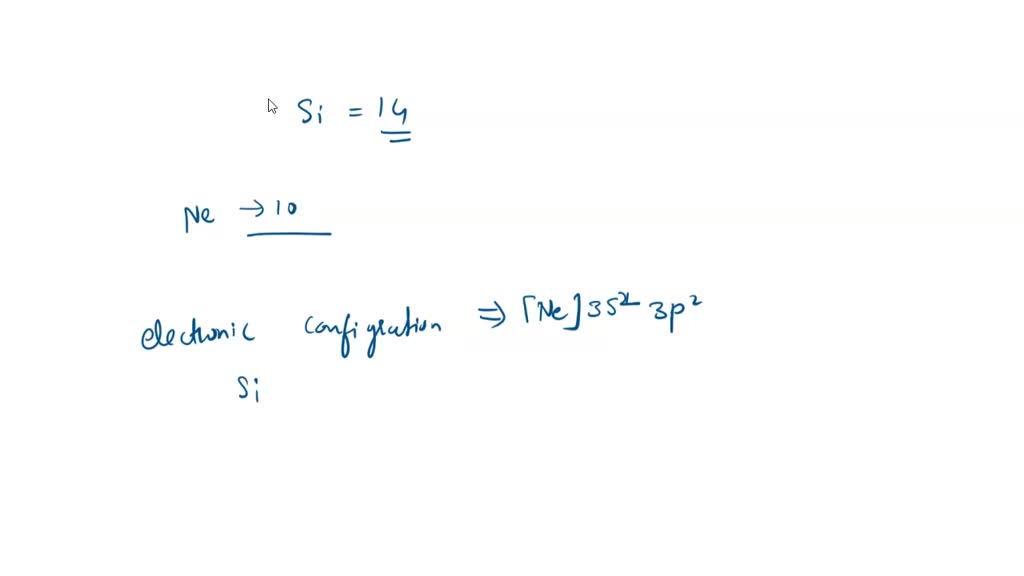SOLVED: 4a) Using Valence Bond Theory, show the hybridization and bonding scheme for silicon tetrachloride (SiCl4): (a) write the atomic orbital diagram for the central atom, (b) circle the atomic orbitals that
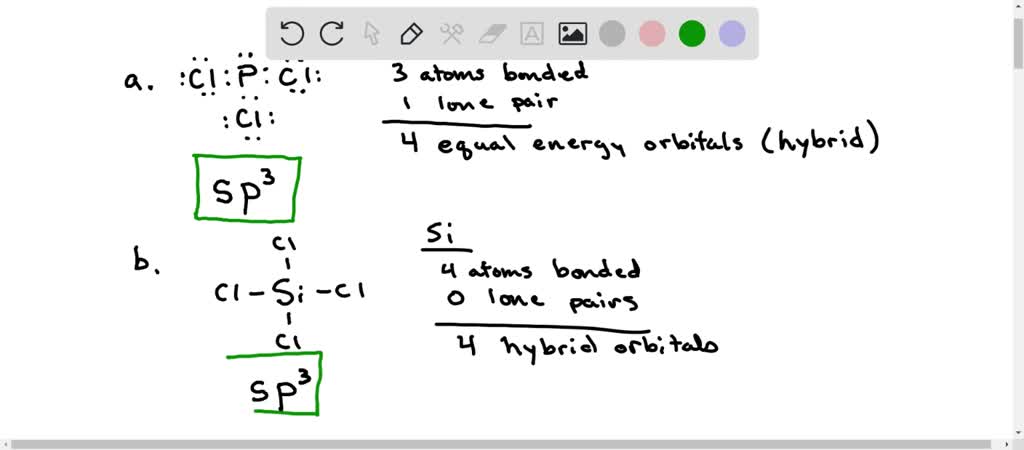
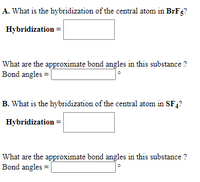
SOLVED: 10.50. What hybrid orbitals would be expected for the central atom in each of the following molecules or ions? a. PCl3 b. SiCl4 c. BeF2 d. SO2

Total number of molecules which hydrolysed at room temperature and hybridization of central atom ... - YouTube