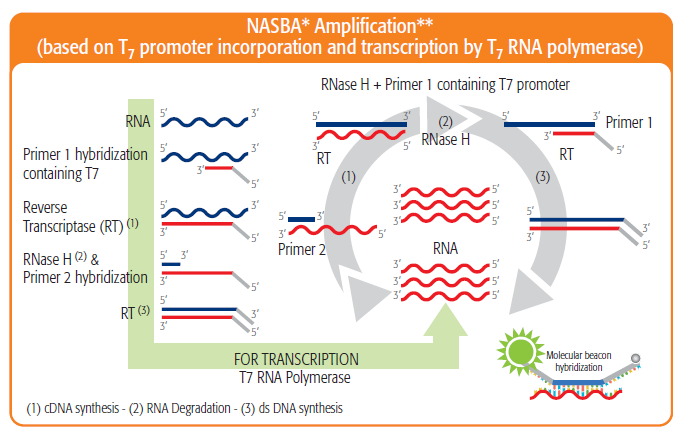
PDF) Evaluation of the bioMérieux VIDAS HIV Duo Quick and Anti-HCV assays for dried blood spot based serosurveillance

Evaluation of the diagnostic performance and optimal cutoff value of a fourth-generation ELISA, VIDAS HIV-1/2 Duo Ultra assay, in a low-prevalence country - ScienceDirect
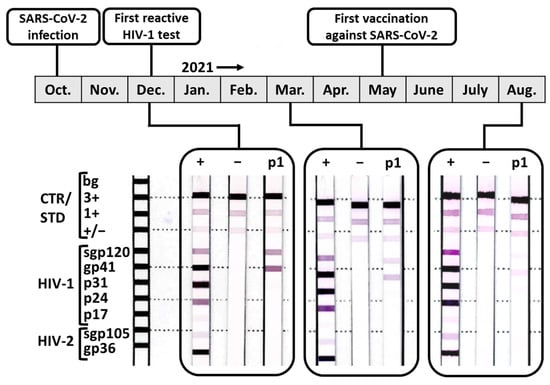
Viruses | Free Full-Text | False-Positive Screening and Confirmatory HIV Diagnostic Test in a Patient with Cured SARS-CoV-2 Infection Is Not Mediated by Env/Spike Cross-Reactive Antibodies

(PDF) Evaluation of a New Combined Antigen and Antibody Human Immunodeficiency Virus Screening Assay, VIDAS HIV DUO Ultra
PDF) Evaluation of the bioMérieux VIDAS HIV Duo Quick and Anti-HCV assays for dried blood spot based serosurveillance
I had the Vidas HIV duo ultra test on the 23rd and 83rd day. I cannot relax psychologically because my lymph node is swollen. Are the tests enough to close the issue? -

Elisa Testi ( ANTI – HIV Testi ) Kişi HIV ile enfekte olduktan sonra bağışıklık sistemi tarafından virüse karşı antikor üretilir. Anti-HIV. - ppt indir

Prolonged Second Diagnostic Window for Human Immunodeficiency Virus Type 1 in a Fourth-Generation Immunoassay: Are Alternative Testing Strategies Required? | Journal of Clinical Microbiology

LABORATORY DIAGNOSIS OF HIV INFECTION: ROLE OF COMBINED HIV P24 ANTIGEN AND ANTIBODY ASSAY | Semantic Scholar

PDF) Evaluation of the bioMérieux VIDAS HIV Duo Quick and Anti-HCV assays for dried blood spot based serosurveillance
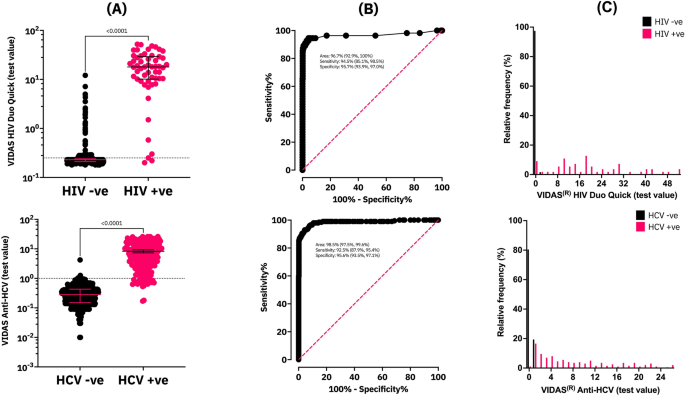
Evaluation of the bioMérieux VIDAS HIV Duo Quick and Anti-HCV assays for dried blood spot based serosurveillance | Scientific Reports

LABORATORY DIAGNOSIS OF HIV INFECTION: ROLE OF COMBINED HIV P24 ANTIGEN AND ANTIBODY ASSAY | Semantic Scholar
![PDF] A Human Immunodeficiency Virus Screening Algorithm to Address the High Rate of False-Positive Results in Pregnant Women in Japan | Semantic Scholar PDF] A Human Immunodeficiency Virus Screening Algorithm to Address the High Rate of False-Positive Results in Pregnant Women in Japan | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/d792306d686814b37c8df9cc937f686f99ee2a22/3-Table1-1.png)
PDF] A Human Immunodeficiency Virus Screening Algorithm to Address the High Rate of False-Positive Results in Pregnant Women in Japan | Semantic Scholar

Evaluation of the diagnostic performance and optimal cutoff value of a fourth-generation ELISA, VIDAS HIV-1/2 Duo Ultra assay, in a low-prevalence country - ScienceDirect